Wharton’s Jelly Reviewed
Wharton’s Jelly
Wharton’s jelly is the gelatinous substance within the umbilical cord. It provides insulation and protection within the umbilical cord. It is a gelatinous substance made largely from mucopolysaccharides. It contains a host of growth factors, cytokines, pathway signaling molecules, and stem cells that have regenerative properties. Wharton’s Jelly also has several other favorable characteristics including strength, flexibility, cushioning, covering, compressibility, and response to friction and shear.
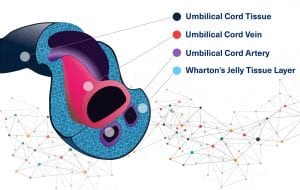 Hyaluronic acid is the most abundant component of the glycosamnioglycans found in the umbilical cord matrix. Hyaluronic acid is also a key factor in the viscoelastic properties in synovial fluid of joints.
Hyaluronic acid is the most abundant component of the glycosamnioglycans found in the umbilical cord matrix. Hyaluronic acid is also a key factor in the viscoelastic properties in synovial fluid of joints.
Wharton’s jelly provides tissue support within the umbilical cord blood, however the FDA has ruled that Wharton’s Jelly is not a homologous derivative for joints, tendon, ligament, and other musculoskeletal applications. As result, it is likely that you will be hearing less about this regenerative medicine alternate, at least for awhile.
In the United States Wharton’s jelly derived mesenchymal stem cells are harvested, along with other growth enhancing factors, from full term neonates with written consent of the family. FDA Guidelines have stated that, among other things, processing cannot alter the original relevant characteristics of the tissue relating to the tissue’s utility for reconstruction and repair, and that replacement and processing cannot alter the relevant biological characteristics of host cells or tissues.
Since tissues that physically support or serve as a barrier or conduit, or connect, cover, or cushion in the donor are generally considered structural tissues for the purposes of determining the applicable regulatory definition, unprocessed Wharton’s jelly would seem to be a regulatory fit, however quite often Wharton’s Jelly is processed by the manufacturer prior to use. That means you need to be careful that any Wharton’s Jelly product is compliant before considering its use.
It is quite fair to say that processing and purification procedures today have to follow strict FDA guidelines. While such regulation can at times interfere with innovation, they do provide the public with a level of comfort, safety, and transparency as well. Since Wharton’s Jelly clinical results to date have been promising, as time goes on you may be hearing more about its use for regenerative medicine applications. Ongoing research is robust and we suspect that this trend will continue.